Due to their advantageous properties, organic semiconductors could be very promising photocatalysts for producing solar fuels. In fact, these materials can be synthetically tuned to absorb visible light, while simultaneously retaining energy levels that are desirable for driving various processes. While photocatalysts based on organic semiconductors have attained promising results, the understanding of the physics underpinning their functioning is still relatively limited.
Researchers at King Abdullah University of Science and Technology (KAUST), Imperial College London and the University of Oxford have been trying to develop organic semiconductor-based photocatalysts that can efficiently harvest solar energy and could thus be used to produce hydrogen more sustainably. Their most recent paper, published in Nature Energy, shows that heterojunction organic semiconductor nanoparticles can generate remarkably long-lasting reactive charges, thus they could efficiently drive sacrificial hydrogen evolution.
"We chose to use organic semiconductors to fabricate our photocatalysts because their bandgaps can be synthetically tuned to absorb strongly in the visible spectrum," Jan Kosco, one of the researchers who carried out the study, told TechXplore. "All else being equal, the more light a photocatalyst absorbs, the more efficiently it can convert solar energy to hydrogen."
Most stable photocatalysts fabricated from inorganic semiconductors, such as TiO2 and SrTiO3 almost exclusively absorb UV wavelengths and have little to no activity under visible light. This can be problematic, as less than 5% of solar energy is carried via UV wavelengths. This fundamentally limits the efficiency of these inorganic semiconductor-based photocatalysts to less than 5%.
Kosco and his colleagues set out to explore the potential of organic semiconductors for driving hydrogen evolution and the photophysics underpinning their functioning further. Their study builds on their previous work on bulk heterojunction organic semiconductor nanoparticle photocatalysts.
"It is important to develop photocatalysts that are active under a broad range of UV-visible-infrared wavelengths to maximize solar light absorption," Kosco explained. "We were initially surprised when we saw that the PM6:PCBM nanoparticles displayed higher H2 evolution rates than the PM6:Y6 NPs."
When they first started conducting their experiments, Kosco and his colleagues expected to find that PM6:Y6 nanoparticles were more active than PM6:PCBM nanoparticles, as Y6 is known to absorb significantly more of the solar spectrum than PCBM. However, when they measured the external quantum efficiencies (EQEs) of PM6:Y6 and PM6:PCBM nanoparticles, they found that the latter are able to convert a larger fraction of the solar energy they do absorb to charges that produce hydrogen.
"In other words, we found that the PM6:PCBM nanoparticles have higher EQEs," Kosco said. "This enables them to produce more hydrogen than PM6:Y6 nanoparticles, even though they absorb less light."
After they measured the nanoparticles' EQEs, Kosco and his colleagues probed them using a series of ultrafast and operando spectroscopic methods. Their hope was to uncover the mechanisms underpinning the higher EQEs they observed in PM6:PCBM nanoparticles.
"We used these techniques to track the photophysical processes responsible for the conversion of photons to catalytically active charges over timescales ranging from picoseconds to seconds," Kosco said. "These methods revealed that the PM6:PCBM nanoparticles are better at converting absorbed photons to long-lived catalytically active charges, and we believe that this is the key reason for their high H2 production efficiency."
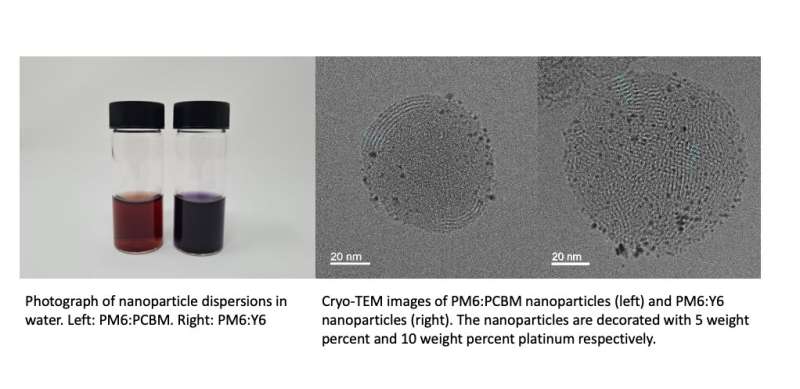
Kosco and his colleagues also imaged the nanoparticles using cryogenic transmission electron microscopy (Cryo-TEM). This is an advanced electron microscopy technique that allows researchers to rapidly freeze a sample and capture images of it under cryogenic conditions, thus preserving its native structure. Using Cryo-TEM, the team was able to generate images of the nanoparticles that clearly captured their internal morphology with a nanometer scale resolution, while they were suspended in vitrified water.
"We expected that charges would form in our nanoparticles, because of the type II heterojunction present inside them," Kosco explained. "However, we were not expecting the charges to 'live' so long inside the nanoparticles. Photogenerated charges typically recombine on the microsecond timescale, but we observed charges in our nanoparticles even a few seconds after photoexcitation."
The lifetime of the photogenerated charges that the researchers observed in their experiments is extremely long compared to that typically exhibited by organic semiconductors. This remarkably long lifetime could be the main factor underpinning their high performance, as it extends the time for the charges to take part in redox reactions at the nanoparticle surface that are known to be relatively slow.
"We hope that this new class of highly active organic semiconductor photocatalysts will accelerate the development of efficient visible light active hydrogen evolution photocatalysts for overall water splitting Z-schemes," Kosco said. "In a water splitting Z-scheme a hydrogen evolution photocatalyst is coupled to an oxygen evolution photocatalyst and together the two photocatalysts drive overall water splitting to H2 and O2. This is analogous to how photosystem 1 and photosystem 2 convert sunlight to chemical energy during photosynthesis in green plants."
In the future, the promising photocatalysts identified by Kosco and his colleagues could be used to create new and better performing solar fuel technologies. While the researchers have so far primarily evaluated their potential for driving the hydrogen evolution reaction, to be applied in real-world settings this reaction should be coupled to oxygen evolution processes, to split water into H2 and O2.
"We are now continuing to develop photocatalysts for H2 evolution, O2 evolution and CO2 reduction to synthetic fuels," Kosco added.
Jan Kosco et al, Enhanced photocatalytic hydrogen evolution from organic semiconductor heterojunction nanoparticles, Nature Materials (2020). DOI: 10.1038/s41563-019-0591-1
© 2022 Science X Network
Citation: Organic semiconductor-based nanoparticles with long-lasting reactive charges (2022, April 11) retrieved 11 April 2022 from https://ift.tt/BJVoOSL
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without the written permission. The content is provided for information purposes only.
"lasting" - Google News
April 11, 2022 at 08:30PM
https://ift.tt/BJVoOSL
Organic semiconductor-based nanoparticles with long-lasting reactive charges - Tech Xplore
"lasting" - Google News
https://ift.tt/gWzK4a6
Shoes Man Tutorial
Pos News Update
Meme Update
Korean Entertainment News
Japan News Update
Bagikan Berita Ini














0 Response to "Organic semiconductor-based nanoparticles with long-lasting reactive charges - Tech Xplore"
Post a Comment