The transient receptor potential vanilloid (TRPV1) ion channel is a polymodal nociceptor broadly expressed in sensory neurons involved in pain sensation, thereby playing an essential role in analgesia. The outer pore domain of TRPV1 is essential for regulation of this channel by temperature, protons and other toxin peptides, so rationally engineering modulators targeting the outer pore of TRPV1 is a potential strategy for regulating pain responses.
On January 3, Yang Fan at the Zhejiang University School of Basic Medical Sciences, Xu Zhenzhong at the Zhejiang University School of Brain Science and Brain Medicine, and Qi Yunkun at the Qingdao University School of Pharmacy co-published a research article titled "Structure-guided peptide engineering of a positive allosteric modulator targeting the outer pore of TRPV1 for long-lasting analgesia" in the journal Nature Communications.
In this study, the researchers rationally engineered a positive allosteric modulator (PAM) s-RhTx targeting the outer pore of TRPV1, discovered the molecular mechanism for the binding of sRhTx and TRPV1, and observed the long-lasting analgesic effect of s-RhTx in pain model mice.
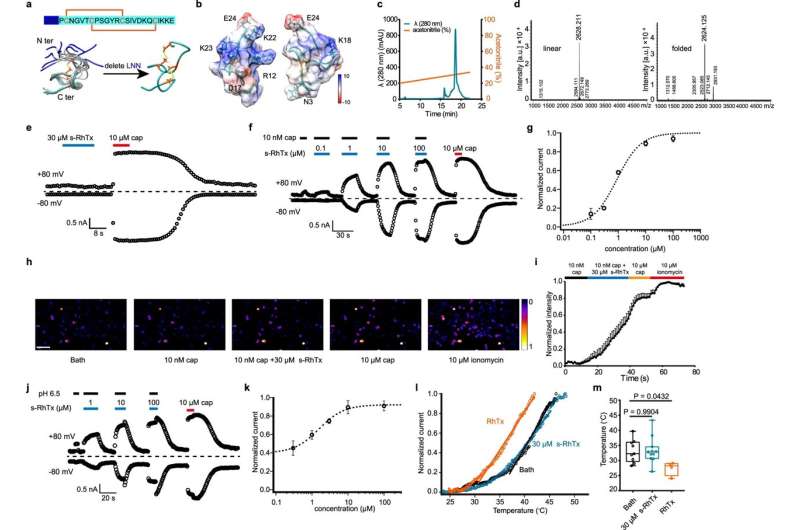
To develop a modulator targeting the outer pore of TRPV1, the researchers engineered a peptidic PAM s-RhTx based on RhTx, a potent TRPV1 agonist from the venom of the Chinese red-head centipede. In contrast to RhTx, s-RhTx can no longer activate TRPV1 as designed. However, it can selectively enhance the currents of TRPV1 evoked by capsaicin and protons in a concentration-dependent manner without altering the heat activation threshold of TRPV1, indicating that s-RhTx is a PAM of TRPV1.
At the molecular level, the researchers calculated the interactions between s-RhTx and TRPV1 and revealed the critical role of E649 and E652 in the outer pore domain for the activity of s-RhTx via molecular docking, electrophysiology and calcium imaging. Moreover, the researchers performed the thermodynamic mutant cycle to show that E652 in the outer pore of TRPV1 specifically interacted with R12 and K22.
In the presence of calcium ions, TRPV1 was desensitized rapidly when activated by capsaicin, while s-RhTx significantly slowed down the capsaicin-induced desensitization of TRPV1, thereby resulting in the death of TRPV1-expressing cells due to calcium overload. This suggested that the combination of low doses of capsaicin and s-RhTx could induce the degeneration of intra-epidermal nerve fiber (IENF) expressing TRPV1 channels and therefore play a crucial role in pain relief.
To test this hypothesis, the researchers assessed the analgesic effect of s-RhTx in naive and inflammatory-pain model mice by administering both s-RhTx and low-doses of capsaicin. The results showed that this strategy could produce a long-lasting analgesic effect by promoting the reversible degeneration of IENF.
The analgesic effects of a single injection of s-RhTx could last for more than three weeks. Remarkably, the local delivery of s-RhTx did not cause any change in the body temperature of the mice, thus overcoming the side effect of commonly used TRPV1 inhibitors in affecting the body temperature.
This study identified the development of a positive allosteric modulator targeting the outer pore of TRPV1 as an immensely promising analgesic strategy. Not only did it report s-RhTx as an analgesic agent, but it also proposed two critical residues in the TRPV1 channel for interactions with s-RhTx, thereby laying a foundation for future development of modulators targeting TRPV1.
More information: Heng Zhang et al, Structure-guided peptide engineering of a positive allosteric modulator targeting the outer pore of TRPV1 for long-lasting analgesia, Nature Communications (2023). DOI: 10.1038/s41467-022-34817-1
Provided by Zhejiang University
Citation: TRPV1 targeted for long-lasting pain relief by allosteric modulator (2023, January 16) retrieved 16 January 2023 from https://ift.tt/Xtkc20B
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without the written permission. The content is provided for information purposes only.
"lasting" - Google News
January 17, 2023 at 12:51AM
https://ift.tt/9CBAW4D
TRPV1 targeted for long-lasting pain relief by allosteric modulator - Phys.org
"lasting" - Google News
https://ift.tt/vKxT0WE
Shoes Man Tutorial
Pos News Update
Meme Update
Korean Entertainment News
Japan News Update
Bagikan Berita Ini














0 Response to "TRPV1 targeted for long-lasting pain relief by allosteric modulator - Phys.org"
Post a Comment